1.
Ozone layer depletion results in
melting of ice at the poles.
increase in number of storms.
skin cancer in humans.
loss of productive farmland.
2.
Which of the following factors can affect the boiling and freezing points of water?
Surface tension
Adhesive forces
Impurities
Density
3.
Purple-leaf colouration in plants is a symptom of deficiency in
magnesium.
nitrogen.
phosphorus.
potassium.
4.
Which of the following diseases in humans is caused by bacteria?
Hepatis B
Herpes
Chlamydia
Prostate
5.
The part of the human ear responsible for amplification of sound is
cochlea.
ossicles.
sacculus.
utriculus.
6.
The general formula of alkyne is
CnH2n+2
CnH2n
CnH2n-2
CnHn
7.
Which of the following bones is present in the forelimb of man?
Femur
Radius
Ribs
Sternum
8.
Minerals included in the layer ration for egg shell formation are calcium and
magnesium.
nitrogen.
phosphorus.
potassium.
9.
Carrot is a good source of which of the following vitamins?
Vitamin A
Vitamin B complex
Vitamin C
Vitamin D
10.
The major reason why relative atomic mass of some elements is not a whole number is the existence of
isomerism.
allotropy.
isotopy.
isotonism.
11.
Which energy transformation occurs in a light emitting diode?
Light energy to chemical energy
Chemical energy to heat energy
Electrical energy to heat energy
Electrical energy to light energy
12.
Which of the following characteristics are associated with local breeds of fowl?
I. Resistant to diseases
II. High foraging ability
III. Susceptible to adverse weather conditions
I and II only
I and III only
I and III only
I, II and III
13.
Temporary hardness of water is removed by heating because
it is caused by insoluble salts.
the salts causing the hardness become soluble.
calcium hydrogen trioxocarbonate (IV) in water is decomposed by heat.
calcium trioxocarbonate (IV) is produced.
14.
Examples of continuous variation include:
I. weight,
II. rhesus factor,
III. sickle cell.
I only
I and II only
I and III only
II and III only
15.
The number of protons in an ion with a charge of +3 is 13. How many electrons are present in the neutral atom?
10
13
16
20
16.
Metals are normally used as electrical wires because they are
brittle.
ductile.
elastic.
malleable.
17.
One advantage of male circumcision is that, it
allows for easy penetration in the vagina.
keeps the penis free from germs.
makes the man sexually active.
makes the man sexually inactive.
18.
Organic manures are preferred to inorganic manures because, they are
easy to apply.
readily available to plants.
relatively cheap.
toxic to plants.
19.
Calculate the number of moles in 5.85 of NaCl.
[Na = 23; Cl = 35.5]
0.01
0.10
1.00
10.00
20.
The diagram below illustrates a ray of light incident on a plane mirror MM'.
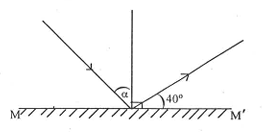
The value of α is
40°
50°
80°
100°
21.
A respiratory surface must have
I. rich supply of blood capillaries.
II. thin wall.
III. large surface area.
Which of the statements above is/are correct?
I only
I and II only
II and III only
I, II and III
22.
In which of the following processes is plant nutrient in the soil lost through gravity?
Capillarity
Erosion
Evaporation
Leaching
23.
A body of mass 20 kg falls from a height of 10 m. If g = 10.0 ms-2, calculate the velocity of the body just before it touches the ground.
10.0 ms-1
14.0 ms-1
20.0 ms-1
24.3 ms-1
24.
A solution of 250 cm3 HCl has a concentration of 2 mol dm-3. Determine the number of moles of the acid.
0.25 moles
0.50 moles
1.00 moles
1.50 moles
25.
In which of the following blood vessels in the mammalian body would high levels of urea be found?
Hepatic artery
Hepatic vein
Pulmonary artery
Pulmonary vein
26.
Land breeze occurs when
cool wind blows across the land during the day.
warm air rises up from the sea during the day.
cool wind blows across the sea at night.
warm air rises up from the land during the day.
27.
The following statements are characteristics of large-scale industries except
volumes of products turned out are large.
many people are involved in its operation.
capital investment is large.
space of operation is limited.
28.
Muscles are mostly made up of
proteins.
starch.
fibre.
cellulose.
29.
Determine the volume of water required to change the concentration of 100 cm3 HCl from 0.5 mol dm-3 to 0.1 mol dm-3.
100 cm3
200 cm3
400 cm3
500 cm3
30.
A force moves a body through a distance of 15 m in the direction of the force. If the work done by the force is 45.0 J, determine the magnitude of the force.
3.00 N
3.15 N
6.00 N
6.75 N
31.
Which of the following statements are functions of soil air?
I. It is for respiration of plant roots.
II. It is for respiration of soil fauna.
III. It is for seed germination.
I and II only
I and III only
II and III only
I, II and III
32.
Digestion of food is completed in the
small intestine.
large intestine.
stomach.
rectum.
33.
Transistors can be used as
I. amplifiers.
II. switches.
III. temperature controls.
Which of the statements above is/are true?
I only
II only
I and II only
II and III only
34.
Sedimentary rocks are not resistant to weathering because, they
are formed from molten matter.
formed from other rocks.
have lines of weakness between mineral aggregates.
have lines of weakness between strata.
35.
The reaction represented by the equation
RCOOH + R'OH ⇋ RCOOR' + H2O is an example of
esterification.
hydrolysis.
neutralization.
oxidation.
36.
All the following crops require staking as a cultural practice except
cowpea.
cucumber.
tomato.
yam.
37.
Which of the following lights will combine with red light to give white light?
Cyan
Green
Magenta
Yellow
38.
Which of the following organelles is called the power house of the cell?
Golgi complex
Mitochondrion
Nucleus
Endoplasmic reticulum
39.
The IUPAC name of the compound Mg(HCO3)2 is
magnesium hydrogen trioxocarbonate (III).
magnesium hydrogen trioxocarbonate (IV).
magnesium hydrogen trioxocarbonate (V).
magnesium hydrogen trioxocarbonate (II).
40.
Which of the following brooder house equipment confines day-old chicks?
Feed trough
Chick guard
Hover
Liter
41.
Symbiotic association in the root nodules occurs in
cocoyam.
millet.
rice.
soya bean.
42.
The method of fertilizer application which is most labour intensive and time-consuming is the
broadcasting method.
drilling method.
foliar method.
ring method.
43.
An electric kettle rated 1.5 kW operates at 200 V. Determine the current passing through the kettle.
7.5 A
133.3 A
201.5 A
300.0 A
44.
The best way of storing yam tubers is
burying them in the soil.
keeping them in silos.
packing them on wooden platforms and in barns.
leaving them in the mounds for long periods.
45.
Blood pressure is highest at
pulmonary veins.
vena cava.
aorta.
ventricular diastole.
46.
The main constituent of natural gas is
ethane.
helium.
methane.
nitrogen.
47.
The SI unit of light intensity is
ampere.
candela.
kilogram.
kelvin.
48.
A 250 cm3 of 0.10 mol dm-3 of KOH is to be prepared. Calculate the mass of KOH required.
[K = 39, O = 16, H = 1]
1.0 g
1.4 g
7.0 g
14.0 g
49.
The male and female sex cells are collectively called
foetus.
gamete.
seed.
zygote.
50.
A characteristics of sound that differentiates the same note played on different instruments is
frequency.
loudness.
pitch.
quality.
(a)
(i)
List three major types of weathering.
(ii)
Explain the role of temperature in weathering of rocks.
(b)
A 100 W heater is connected to a 240 V mains supply. Calculate the:
(i)
current drawn;
(ii)
resistance of the heater.
(c)
(i)
List three compounds which have electrovalent bond.
(ii)
Give two characteristics of electrovalent compounds.
(d)
(i)
What is rhesus factor?
(ii)
Explain briefly how the rhesus factor in humans could result in miscarriage.
(a)
(i)
Explain briefly how solar energy is used to generate electricity.
(ii)
State two other uses of solar energy.
(b)
State five ways of conserving water in the home.
(c)
(i)
State three management practices to ensure high yield in maize production.
(ii)
Explain how two of the management practices stated in (i) help in ensuring high yield of maize.
(d)
State
(i)
two effects of air masses on the environment.
(ii)
three ways by which global warming can be reduced.
(a)
(i)
Explain why aluminium resists corrosion but iron does not.
(ii)
State two methods of preventing iron from rusting.
(b)
(i)
Explain the term culling as used in livestock management.
(ii)
State three benefits of practising culling.
(c)
(i)
Distinguish between grafting and budding.
(ii)
Give three reasons for grafting and budding.
(d)
(i)
State the principle of conservation of energy.
(ii)
State the energy transformation that occur in each of the following devices:
(α)
a moving motorcycle;
(β)
a television set in operation.
(a)
(i)
What are secondary colours of light?
(ii)
Name the colour that results from the combination of each of the following pairs of colour of light:
(α)
red and green;
(β)
blue and green;
(γ)
red and blue.
(b)
(i)
Define the mole of a substance.
(ii)
Calculate the number of atoms in 18 g of magnesium metal.
[Mg = 24, Avogadro number = 6.023 x 1023]
(c)
(i)
Differentiate between bone and cartilage.
(ii)
Name three parts of the human body where cartilages are found.
(d)
(i)
Explain the term deep litter system as used in poultry.
(ii)
Give three advantages of the deep litter system.
(a)
Explain how each of the following actions causes loss of soil nutrients:
(i)
crop removal;
(ii)
continuous cropping;
(iii)
drainage.
(b)
(i)
Give the reason for using each of the following devices in household wiring:
(α)
earthing;
(β)
stabilizer;
(γ)
fuse.
(ii)
Explain the behaviour of a p-n junction diode when it is forward biased.
(c)
State in which way each of the following factors is important in the germination of seeds:
(i)
water;
(ii)
oxygen;
(iii)
warmth;
(iv)
sunlight.
(d)
The table below shows a list of some chemical substances and the sources from which they are obtained but not correctly matched. In a tabular form, match each chemical substance with its correct source.
| Substance | Source | |
| (i) | Ammonia | Lime water |
| (ii) | Potassium hydroxide | Sour palm wine |
| (iii) | Calcium oxide | Green vegetables |
| (iv) | Ethanoic acid | Ashes of plants |
| (iv) | Ascorbic acid | Decomposed organic matter |
(a)
(i)
What is meant by insanitary condition?
(ii)
State three ways by which fire from electrical causes could be prevented.
(b)
(i)
Give three effects of ecto-parasites on cattle.
(ii)
List four methods of controlling ecto-parasites of livestock.
(c)
Describe briefly how a standard solution of sodium hydroxide is prepared in the laboratory.
(d)
(i)
Define displacement.
(ii)
A bird flies with a constant velocity of 100 ms-1 for 10 minutes.
Calculate the magnitude of its displacement.